Adaptive Clinical Trial Design Case Studies. SMi Groups Adaptive Designs in Clinical Trials Conference will return to London on 3rd and 4th April 2017 and will feature two case studies on Bayesian adaptive designs in medical device development. Adaptive designs for medical device clinical studies.
Adaptive Designs For Medical Device Clinical Studies, Evaluation of medical imaging devices often involves clinical studies where multiple readers MR read images of multiple cases MC for a clinical task which are often called MRMC studies. An adaptive design for a medical device clinical study is defined as a clinical study design that allows for prospectively planned modifications based on accumulating study data without. Known as adaptive design the method can minimize clinical trial sponsors resource requirements and increase chances of study success. What makes a randomised clinical trial adaptive.
 Pin On Services From pinterest.com
Pin On Services From pinterest.com
The EMA Reflection paper on methodological issues in confirmatory clinical trials planned with an adaptive design CHMPEWP245902 defines a study design as adaptive if the statistical methodology allows the modification of a design element for example sample-size randomization ratio number of treatment arms at an interim analysis with full control of the. Integrating indacaterol dose selection in a clinical study in COPD using an adaptive seamless design. Today the FDA published the Adaptive Designs for Medical Device Clinical Studies Final Guidance. An adaptive design for a medical device clinical study is defined as a clinical study design that allows for prospectively planned modifications based on accumulating study data without.
What makes a randomised clinical trial adaptive.
Read another article:
There has been considerable interest among pharmaceutical and other medical product developers in adaptive clinical trials in which knowledge learned during the course of a trial affects ongoing conduct or analysis of the trial. An adaptive design is defined as a design that allows modifications to the trial andor statistical procedures of the trial after its initiation without undermining its validity and integrity. Known as adaptive design the method can minimize clinical trial sponsors resource requirements and increase chances of study success. Key study design components can be adapted throughout the trial. An adaptive design for a medical device clinical study is defined as a clinical study design that allows for prospectively planned modifications based on accumulating study data without.
 Source: jliedu.com
Source: jliedu.com
The Food and Drug Administration FDA has started encouraging the use of adaptive designs for clinical studies. The most frequently appearing type of adaptation was the seamless Phase IIIII design 81142 57 followed by adaptive group sequential 30142 21 biomarker adaptive 28142 20 adaptive dose-finding 23142 16 pick-the-winnerdrop-the-loser 13142 9 sample size re-estimation 11142 8 adaptive randomisation 10142 7 adaptive. An adaptive design for a medical device clinical study is defined as a clinical trial design that allows for prospectively planned modifications based on accumulating study data without undermining the trials integrity and validity. Adaptive Designs for Medical Device Clinical Studies. Adaptive Design Clinical Trials Jli Blog.
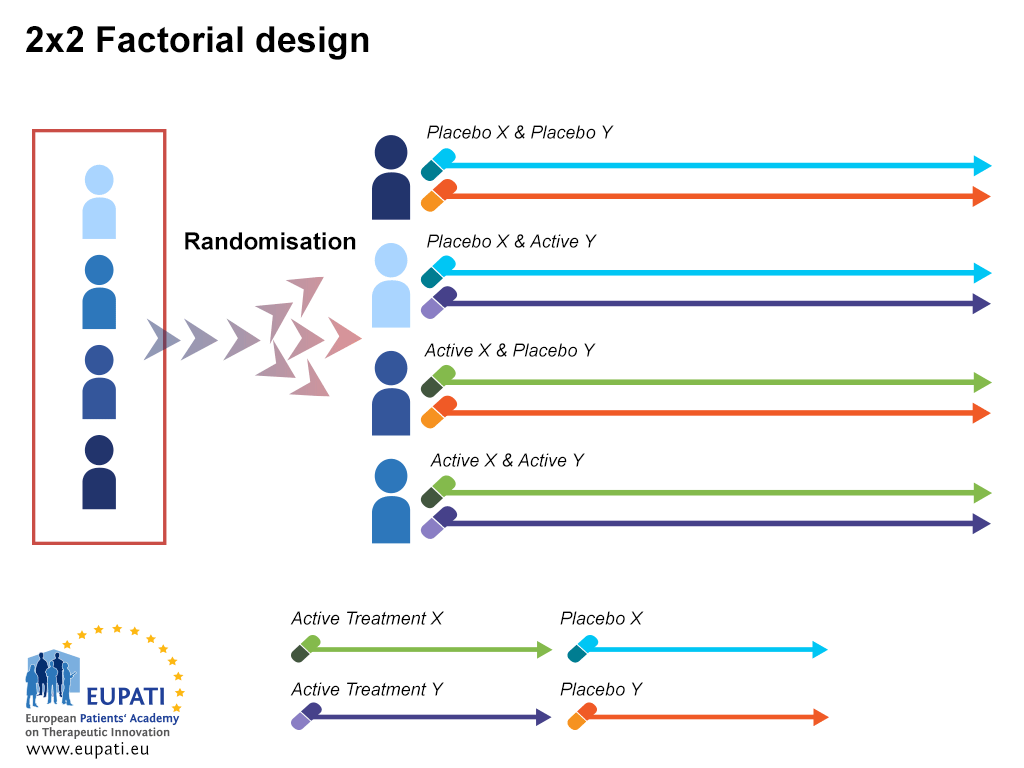 Source: toolbox.eupati.eu
Source: toolbox.eupati.eu
Adaptive Designs for Medical Device Clinical Studies. Today the FDA published the Adaptive Designs for Medical Device Clinical Studies Final Guidance. An adaptive design clinical trial can help to create a stronger value proposition for your medical device and give you a competitive edge by reducing development costs and accelerating time-to-market. The pharmaceutical landscape is evolving as stakeholders pursue the use of real-world evidence precision medicine and complex innovative trial designs CID to increase efficiency of development. Clinical Trial Designs Eupati Toolbox.
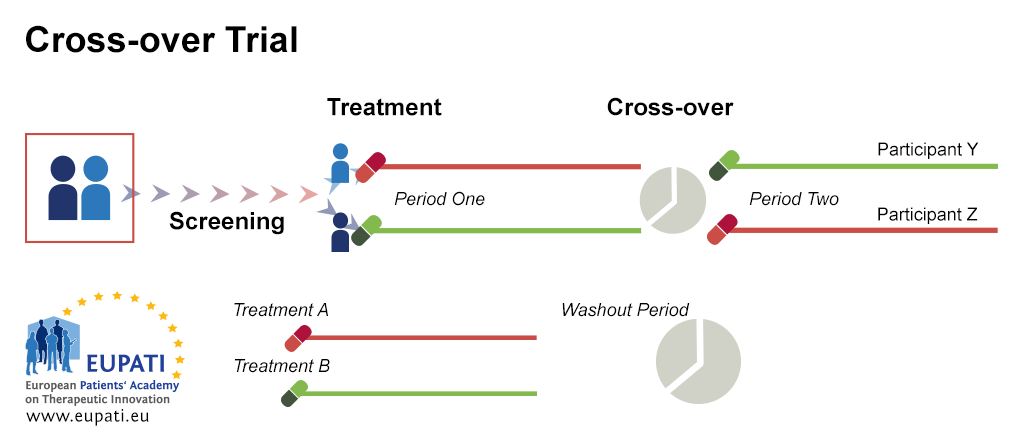 Source: toolbox.eupati.eu
Source: toolbox.eupati.eu
An adaptive design for a medical device clinical study is defined as a clinical trial design that allows for prospectively planned modifications based on accumulating study data without undermining the trials integrity and validity. Today the FDA published the Adaptive Designs for Medical Device Clinical Studies Final Guidance. FDA Finalizes Guidance on Adaptive Designs for Device Studies. Following the release of the FDA draft Guidance document on adaptive. Clinical Trial Designs Eupati Toolbox.
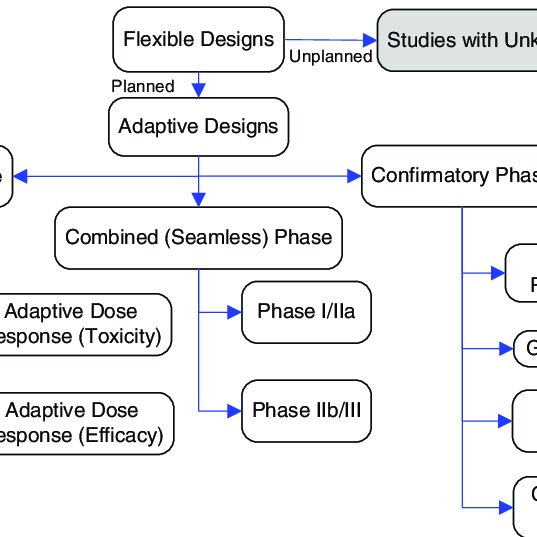 Source: researchgate.net
Source: researchgate.net
Barnes PJ Pocock SJ Magnussen H et al. Known as adaptive design the method can minimize clinical trial sponsors resource requirements and increase chances of study success. The 33-page guidance which finalizes a draft version released for comment in September 2018 and replaces an earlier guidance from 2010 sets out FDAs recommendations on adaptive trial design principles and the information FDA will review from adaptive studies submitted as part of investigational new drug applications INDs new drug applications. An adaptive design for a medical device clinical study is defined as a clinical study design that allows for prospectively planned modifications based on accumulating study data without. Summary Of Different Types Of Adaptive Designs For Clinical Trials Download Scientific Diagram.
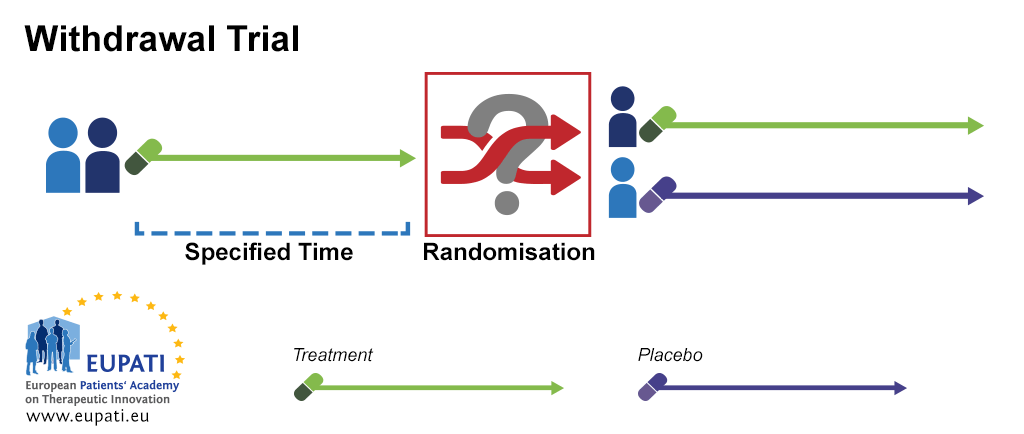 Source: toolbox.eupati.eu
Source: toolbox.eupati.eu
Adaptive Designs for Medical Device Clinical Studies. Consequences and gains of possible trial adaptations need to be understood before initiation. This final guidance applies to premarket medical device submissions including premarket approval applications PMA premarket notification. By spelling out when adaptive designs are acceptable in clinical trials for devices requiring Premarket Approval PMA or 510k premarket notification the FDA seeks to better inform both device manufacturers and its own. Clinical Trial Designs Eupati Toolbox.
 Source: clinicaltherapeutics.com
Source: clinicaltherapeutics.com
An adaptive design for a medical device clinical study is defined as a clinical trial design that allows for prospectively planned modifications based on accumulating study data without undermining the trials integrity and validity. It provides clarity on how to plan and implement adaptive designs for clinical studies used in medical device development. Analytical results have been derived. An adaptive design clinical trial can help to create a stronger value proposition for your medical device and give you a competitive edge by reducing development costs and accelerating time-to-market. The Evolution Of Master Protocol Clinical Trial Designs A Systematic Literature Review Clinical Therapeutics.
 Source: jliedu.com
Source: jliedu.com
It provides clarity on how to plan and implement adaptive designs for clinical studies used in medical device development. Consequences and gains of possible trial adaptations need to be understood before initiation. This final guidance applies to premarket medical device submissions including premarket approval applications PMA premarket notification. An adaptive design for a medical device clinical study is defined as a clinical trial design that allows for prospectively planned modifications based on accumulating study data without undermining the trials integrity and validity. Adaptive Design Clinical Trials Jli Blog.
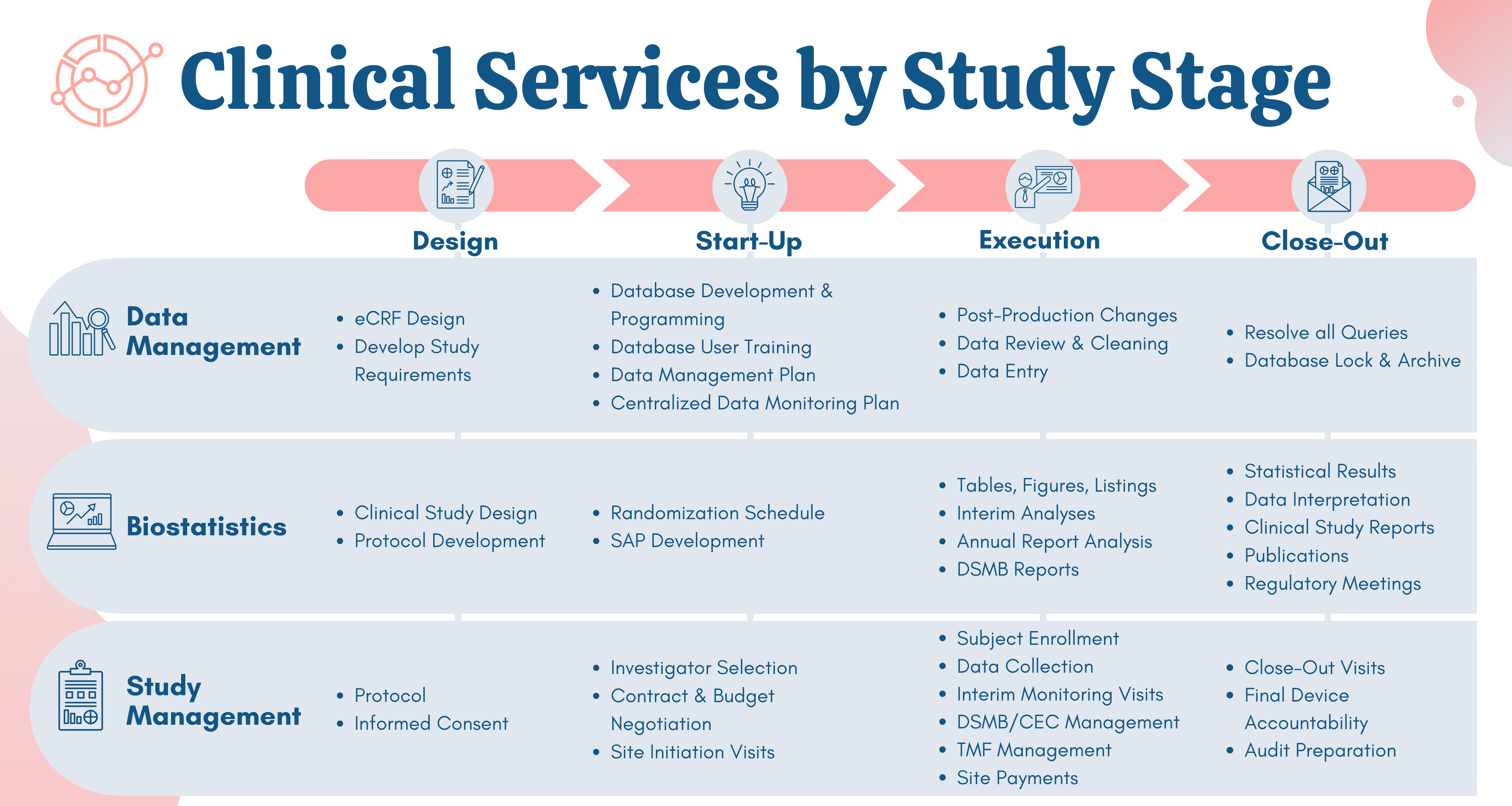 Source: namsa.com
Source: namsa.com
The faster path to market. Integrating indacaterol dose selection in a clinical study in COPD using an adaptive seamless design. Pulm Pharmacol Ther 2010 23. Adaptive design for drug and device studies. Medical Device Clinical Research Namsa.
 Source: pinterest.com
Source: pinterest.com
What makes a randomised clinical trial adaptive. The purpose is to. The pharmaceutical landscape is evolving as stakeholders pursue the use of real-world evidence precision medicine and complex innovative trial designs CID to increase efficiency of development. Adaptive design for medical devices. Pin Na Doske Interface Design.
 Source: pinterest.com
Source: pinterest.com
An adaptive design is defined as a design that allows modifications to the trial andor statistical procedures of the trial after its initiation without undermining its validity and integrity. We develop adaptive MRMC design methodologies to enable study resizing. The Food and Drug Administration FDA has started encouraging the use of adaptive designs for clinical studies. Integrating indacaterol dose selection in a clinical study in COPD using an adaptive seamless design. Pin On Services.
 Source: pinterest.com
Source: pinterest.com
Key study design components can be adapted throughout the trial. Bringing in voices from the industrys best clinical specialists strategists and statisticians SMis 9th Adaptive Designs in Clinical Trials conference will feature case. This final guidance applies to premarket medical device submissions including premarket approval applications PMA premarket notification. An adaptive design is defined as a design that allows modifications to the trial andor statistical procedures of the trial after its initiation without undermining its validity and integrity. Pin On Farmacevtski Proizvodi.
 Source: cancertreatmentreviews.com
Source: cancertreatmentreviews.com
The pace of the uptake of adaptive designs in clinical research however has remained well behind that of the statistical literature introducing new methods and highlighting their potential advantages. It provides clarity on how to plan and implement adaptive designs for clinical studies used in medical device development. This final guidance applies to premarket medical device submissions including premarket approval applications PMA premarket notification. In particular we resize the study and adjust the critical value for hypothesis testing simultaneously after an interim analysis to achieve a target power and control the type I error rate in comparing AUCs of two modalities. New Clinical Trial Designs In The Era Of Precision Medicine An Overview Of Definitions Strengths Weaknesses And Current Use In Oncology Cancer Treatment Reviews.
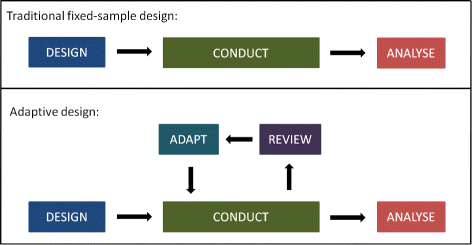 Source: bmcmedicine.biomedcentral.com
Source: bmcmedicine.biomedcentral.com
The EMA Reflection paper on methodological issues in confirmatory clinical trials planned with an adaptive design CHMPEWP245902 defines a study design as adaptive if the statistical methodology allows the modification of a design element for example sample-size randomization ratio number of treatment arms at an interim analysis with full control of the. What makes a randomised clinical trial adaptive. In essence adaptive designs allow prospectively planned modifications to a clinical trial based on interim data provided scientific validity the ability to draw sound inferences and data integrity credibility and reproducibility are preserved. An adaptive design for a medical device clinical study is defined as a clinical trial design that allows for prospectively planned modifications based on accumulating study data without undermining the trials integrity and validity. Adaptive Designs In Clinical Trials Why Use Them And How To Run And Report Them Bmc Medicine Full Text.
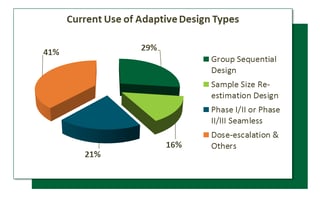 Source: veristat.com
Source: veristat.com
The faster path to market. In particular we resize the study and adjust the critical value for hypothesis testing simultaneously after an interim analysis to achieve a target power and control the type I error rate in comparing AUCs of two modalities. FDA Finalizes Guidance on Adaptive Designs for Device Studies. We develop adaptive MRMC design methodologies to enable study resizing. What Are The Major Common Types Of Adaptive Designs Used In Clinical Trials Today.
 Source: pinterest.com
Source: pinterest.com
Analytical results have been derived. This final guidance applies to premarket medical device submissions including premarket approval applications PMA premarket notification. Adaptive Designs for Medical Device Clinical Studies. The most frequently appearing type of adaptation was the seamless Phase IIIII design 81142 57 followed by adaptive group sequential 30142 21 biomarker adaptive 28142 20 adaptive dose-finding 23142 16 pick-the-winnerdrop-the-loser 13142 9 sample size re-estimation 11142 8 adaptive randomisation 10142 7 adaptive. 10 Must Haves For Home Health Occupational Therapy Myotspot Com Orthopedics Occupational Therapy Occupational Therapist.